Remember LNP member Craig Kelly foolishly claimed on “sceptic” blog Watts up with that that Australia’s record-breaking temperature was not an anomaly? According to Kelly things were hotter back in 1790s:
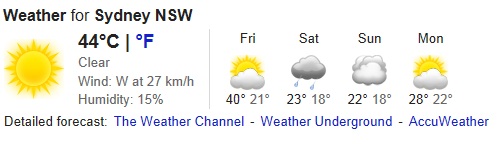
It’s been a scorcher. With the mercury soaring to 42.3 C in Sydney last week and the city in meltdown, the papers screamed, “This is climate change. It is here. It is real.” Even the taxpayer-funded Climate Commission could not hide their excitement declaring, “it was hotter than before” and that “climate change” was responsible for the “unprecedented” extreme heat Sydneysiders were experiencing…
For while the mercury peaked at 42.3 C last Tuesday at Observatory Hill in Sydney – more than 222 years ago at 1.00pm on the 27th Dec 1790 (measured at a location just stones-throw from Observatory Hill) the mercury hit 108.5 F (42.5 C) before peaking at 109 F (42.8 C) at 2.20pm.
Craig – Sydney just hit 44.9c (oops correction its passed 45c).
Heat records are being broken today. The highest recorded temperature for Sydney was actually January 14 1939 when the mercury reached 45.3 at the Observatory Hill station. We’ve just seen the second highest recorded temperature for Sydney.
One hope’s Craig will do a follow-up post for Watts up with that – I’m sure it would make interesting reading.
Hi,
It is 47 degrees here at Wingham NSW at the moment.
Wow… I’ve never experienced that kind of heat before. Stay cool!
Hi,
Now 47.3 degrees. That is with our temp sensor in the shade btw.
Our Air Con has dragged it down to 32.7 inside.
Took the sensor out into the sun, immediately jumps up to 53.4 degrees
The humidity is only 20% though.
The record temperature has been equaled at Obs Hill. 46.5C max so far at Penrith on the city’s western fringe.
45.7C now. Record broken.
45.8C
http://www.abc.net.au/news/2013-01-18/temperature-in-sydney-soars/4471424
record
Craig Kelly has emailed me and wants to learn, we will take it slowly and hopefully get there
none of the correspondence with him will be posted anywhere
john byatt
Well done John!
Use words of few syllables.
You guys don’t get it!
Sure, the record was broken by 0.3C. Earth shattering! Sydney can also be as cold as 18C in January. 45C is the normal expected maximum temp for this time of year. Are you guys arguing about climate or weather? If it is climate, explain the “climate” being experienced in Britain!
Soon enough, you guys are going to have to admit the drivers of “Climate” is the sun and the moon. Always has, always will!
Gas laws cannot be broken. Thermo laws cannot be broken. A cooler object cannot warm a warmer object.
A realist trucky!
The moon drives climate? Pray tell, how?
Roy spencer has a post on Thermo Laws, as he is a sceptic perhaps you will listen to roy?
bet you are a creationist with such absurd remarks
http://www.drroyspencer.com/2010/07/yes-virginia-cooler-objects-can-make-warmer-objects-even-warmer-still/
No record cold being set in Britain. Record heat being set in Australia. That’s not a draw.
Globally heat records are being set three times more quickly than cold records. The climate is warming. It’s due to man’s emissions of CO2.
We have a nutter in QLD who claims that the super moon means more rain for Gympie, do not know why it picks on gympie?
http://www.gympietimes.com.au/news/could-the-moon-cause-more-flooding/772722/
Wow. That moonie-floodie thing has me convinced.
If you want to see a flock of loons, go over to Facebook and have a look at https://www.facebook.com/AskAClimateScientist?fref=ts. I’ve blocked nearly 50 of them. As soon as they state there’s a conspiracy they’re gone.
No, no, no! It’s not fair to parody the denialist numbskulls, and certainly not to go completely overboard and fake a knuckle-dragger this grotesque. The denialists make complete fools of themselves without some cruel blighter sending them up even more. Still-the moon bit was a really clever confabulation.
Ah!!! The ‘Gympie Times’. Say no more. The dead heart and head of Dunning-Krugerism in this country. Perhaps we should coin the phrase ‘He/She’s looking/acting/behaving a bit ‘gympie”, for anyone displaying the signs of early dementia, or behaving like a senile delinquent. And, with Abbott still pretty much odds on (as the economy grinds to a halt) we look like getting a ‘ gympie Government’.
No crank the editor of the gympie times Nev Mcharg always prints the latest on climate change science.
He even printed this from me yesterday
Dear Sir,
It will no doubt come as a surprise to Graeme Ellingsen (The Gympie Times Jan 15) that nearly every church in Australia and around the world have statements supporting the science of climate change.
From the Adventists to the Vatican they all call for urgent action to prevent the future catastrophes’ that will first fall upon the poor of the world.
Graeme, it is you and your ilk who are the odd men out. Most churches recognize the role of humanity as stewards of the planet, a responsibility bestowed upon them by their God. Graeme relieves himself of that responsibility and demands that God should be the one responsible for averting the unfolding disaster due to human insanity.
It would appear to me that your God is the God of dispassion. A god who denies the very atmospheric physics that you believe he created, Civilizations have collapsed in the past, and we should try to learn from those events to ensure that we do not follow down the same path of destruction. During times of crises, cults have always arisen claiming that their God would save them, it has not happened in the past and it won’t happen now…
My most ‘umble apologies to the Gympie Times. That’s what you get for stereotyping. Still, if you’ve passed through there, you’d have to admit, that it has its fair share of….Queenslanders! I am incorrigible.
Second law of Thermo states heat can only travel in one direction. Without looking at the site in question, it will be about some BS that when a molecule radiates heat it radiates in all directions. All may sound like solid science but radiation back toward a warmer object isn’t going to make it hotter. Gradient only goes one way. As for the moon? Gravity. Do you actually think the only thing the moon’s gravity effects is ocean? We are surrounded by an ocean of air. It has mass and is equally effected by gravitational forces.
Record? 0.3C on a record from 1939 on a local thermometer. Are you guys so serious that the world is warming dangerously by 0.3C in 74years???? According to your information we are supposed to be 5C hotter in another 30 years….according to Karoly.
And how does the moon’s gravitational pull drive the climate of Earth?
For example, does it tend to make the climate hotter or cooler? Wetter or drier? And what is the mechanism underlying these changes?
Can you cite any peer reviewed research papers to support your claim?
Sorry about the delay…A real example on lunar effects on the atmosphere…The Morning Glory up in the Gulf. Caused by the apparent change of the orbital alignement of the moon from the northern hemisphere to the southern hemisphere….note the word APPARENT. The orbit is still the same but as observed from the Earth the Moon appeares to move south in its orbit very quickly. The effect is a wave of air breaking over Cape York Peninsula.
This happens pretty regularly around spring equinox.
As for the climate…it is just one of those things that too many people argue from both sides. I am curious how with the 18.6 year lunar cycle how it lines up with major flooding along the length of the east coast. Sure we have to coincide with La Nina and negative IOD to get the NW cloud bands to get the floods but oh boy, does it rain!
Pardon me if I’m less than impressed. You stated that the moon was a driver of climate and you have nothing to back up that statement.
why would you get into an igloo to keep warm they are made of bloody ice ?
ROY Spencer sceptic
Andrew says:
July 23, 2010 at 1:42 PM
So basically, the misunderstand is that it is incorrectly thought that it is being said that the cold atmosphere adds heat to the surface. A cold body cannot add (net) heat to a warmer body, but it can inhibit the loss of heat from that body.
Do I have this about right?
Roy W. Spencer, Ph. D. says:
July 23, 2010 at 1:44 PM
yes, exactly.
Mark you need a brain transplant
You guys are misinterpreting the science…Any Mech Engineer can tell you how insulation works. The igloo of ice is still ice and will freeze you with direct contact. The reason it is warm for the inhabitants is the structure inhibits convection. If you are crazy enough to sit or touch the ice without any layering of animal pelts or seal skins you will lose considerable heat to the ice by conduction.
Air is a very poor conductor of heat. The reason insulation batts work in your roof is because the loose weave of the glass inhibits air from moving freely. Heat still transfers through the medium but at a slower rate than just radiation and convection measured in an open space. The weave stops the air moving stopping or slowing convection. The insulation slows the transfer of heat. In winter from the warmer living space into the roof. In summer from the warmer roof into the cooler living space.
The question below to Roy Spence should have continued with…
“Does CO2 add to this effect of slowing the transfer of heat?”
The answer will always be..at the concentrations recorded in the atmosphere, the change in R value of air is almost immeasurable!
I dare anyone to state what increase in R value of air due to increasing CO2 concentrations. Insignificant! Yet, this is what you believe is happening?
Last time I was offered to sleep with an inuit guys wife, we were both nude in the igloo, go figure
Building an igloo in Cape Dorset … (−49 °F), but on the inside the temperature may range from −7 °C (19 °F) to 16 °C (61 °F) when warmed by body heat alone.
ah 16C, great temp for a good shag
No, this is not what “we” believe is happening. Global warming has nothing to do with R values, which represent insulative properties. ‘Greenhouse’ gases have a quite separate radiative property, and it is this property that causes the planet to warm.
Your “dare” is predicated on a completely flawed representation of the energy-transfer action of gases, ‘greenhouse’ and otherwise,
You need to go back to high school and take basic science again.
John, I would need another citation for Spense’s hypothesis. For his hypothesis to work he would have to add more energy to the system. Simply put. If the bar is at a certain temperature supposedly at equilubrium within the vacuum, add the cooler bar. As the cooler bar warms up it causes the heated bar to warm up as well???? This suggests energy added to the system…..Or….an Astronaut up at the ISS, working on the shaded side of the station touches the colder station resulting in his hand warming up??????as his body heat interacts through his suite through contact and the surrounding vacuum of space his hand gets hotter????? EXPLAIN?
Mark roy will be more than willing to indulge your fantasy
So, where do you think you got your analysis wrong, Mark? And first year mecchie would look at the answers at the back of his textbook, see they differ from his, and try to figure it out.
Last time I checked second law, it stated that there exists an extensive function called entropy for which, during a transformation for an *isolated* system, the variation can only be positive. You have several formulations available, the one I wrote is imho the clearest, but none use heat transfert – since heat transferts are described by the first law.
Heat can travel from cold to warm, otherwise you wouldn’t be able to describe fridges.
He goes from claiming the opposite to now claiming that we are saying that CO2 has an R factor,
too dumb to help
You wouldn’t be able to describe fridges!!!!!!????? Are you serious?
First law of Thermo……energy can neither be created or destroyed.
Second law is heat transfer… In one direction only!
The reason your fridges works is because the warmer products frozen in the freezer are WARMER than the refrigerant flowing through the evaporator as a liquid. Clearly, you guys do not understand Enthalpy/Entropy!
That does not describe it.
describe the full process
I do not know about Australia, but in France for engineer studies two direct applications are studied as soon as 2nd law appears in thermodynamic courses :
– the Carnot cycle
– the heat pump
I’m quite frankly surprised. But I made the assumption that Mark undertook mechanical engineering courses, maybe this is where I made a mistake ?
Bratisla, almost got a student exchange job with Seimens (Steam plant/power generation)in your lovely country…I felt I couldn’t learn the language well enough to compliment my study so bailed.. I should have went just for the experience!
Anyway.. Carnot Cycle by four steps-
1 Reversible isothermal expansion…cooling in condensor
2 Insentropic (reverse adiabatic) expansion…work expansion in cylinder
3 Isothermal heat rejection(reversible compression)…heating in evaporator
and
4 Isentropic compression…..compression in cylinder
and again for effect.
In reality the cycle is politropic due to losses in heat and compression
OK Mark. Now describe how a heat pump works; for example, how an air conditioner moves heat from the space it is cooling to the hotter space outside.
Here Mark
Although internal energy will not spontaneously flow from a cold region to a hot region, it can be forced to do so by doing work on the system. Refrigerators and heat pumps are examples of heat engines which cause energy to be transferred from a cold area to a hot area. Usually this is done with the aid of a phase change, i.e., a refrigerant liquid is forced to evaporate and extract energy from the cold area. Then it is compressed and forced to condense in the hot area, dumping its heat of vaporization into the hot area.
You cannot be serious? Basic refrigeration engineering and I have to explain how the cycle works….the reverse Rankin vapour compression cycle to be specific! Compress a refrigerant gas (super heated gas), pass the hot compressed gas through a condensor to remove heat to form a high pressure liquid(saturated)then throttle the liquid through either a capillary tube, Ax or Tx valve. (this is the cool bit about this cycle) The liquid goes through the valve and undergous a rapid abrupt reduction in pressure. This rapid reduction pressure results in adiabatic flash evaporation of a small part of the liquid…auto-refrigeration lowers the temperature of the liquid/vapour mixture to the desired cold temp lower the the target temperature, pass this liquid/vapour mixture through an evaporator. This can either be inside a refrigerator or the cold side of an airconditioner. Heat is removed from either ambient air inside a room or absorbed from the chest of a refrigerator. This heat evaporates the liquid back into a gas. Gas goes to the compressor to complete the cycle….A heat pump…just like a steam engine in reverse!
If you have ever been lucky enough to go through an old style Linde cycle gas liquifaction plant. You would have seen air being compressed cooled and then expanded into a piston driving a wheel then ecompressed and then passed into another piston and repeat for about eight compressions and progressively smaller cylinders until you have got the air down to a temperature after it leaves the last cylinder as liquid oxygen. Various gases have different liquifaction temps so as the mixture cools after doing work the various liquids are drawn off. Oxygen Nitrogen and Argon are the main liquids produced. It is so old world, it is amazing how simple it is. Nowadays they just use a gas turbine and to do the same thing. Same cycle just a different means of compression and expansion.
Everyone would have seen this watching someone operating a compressed air jack-hammer for a long period. After a while the exhaust manifold will have ice forming because the air has been made to do work resulting in a lower temperature. Remember the properties of Carnot reverse adiabatic cycle? As gas expands in a cyclinder, doing work by moving a piston, the temperature drops.
Have to admit, it has been years since I have had to explain what is so obvious.
Yes it even removes the heat from stuff that is at zero C if that is how you want to describe it
step 1 : define the system you study, here the fridge + cooling engine. The fridge is defined by its interior temperature T, and is in contact with an infinite heat reservoir called atmosphere (usually) defined by its temperature Ta.
step 2 : make the thermodynamical cycle work. You bring a work W to the system, in order to transfer heat from the system to the outside.
step 3 : T drops below Ta. For example, outside is a roasting 40°C, but inside the fridge you attain 20°C. However, your objective is to attain Tf (usually 5°C), when the stuff inside will not rot within hours and the beer (belgian one, of course) will be cool enough to help you withstand the summer. No problem, bring more work W, and continue to transfer heat Q from the inside T to the outside Ta. With T<Ta.
You just transferred heat from a place colder than its recipient. And it doesn't violate the second law, because you paid extra for that. And all you need to be convinced is to look at a fridge, because its main purpose is obviously to extract heat from something …
I do not know how to formulate it in a simpler way. Since you seem to know about cycles, do the calculations, calculate the heat transfer between the inside of the fridge to the outside, and see its sign – you will know where the heat goes …
Bratisla…you have to look at the problem from the frame of reference of the working fluid…The refrigerant! NOW to realy play with your mind. In Antarctica, where mid winter temperatures are in the realm of -40C and colder and the freezers inside the buildings are running at -5C. The refrigerators are run like a heat pump…pumping heat energy INTO the freezer to keep it at -5C. The freezer is warmer than the ambient conditions outside the building!
In Taswegia uncle arthur puts da butter in the fridge ta stoppit freezing.
go and talk to roy, this is boring and irrelevant
Is this “really playing with our minds” because the second law is being “broken”?
Or do you just not realise that you are describing a system which moves heat from a colder body to a warmer one?
mark, I look at the problem from wherever I want, and what is of interest for me is the interior of the fridge, where my Chimay lies. I tend to think I know how you can describe the refrigerant cycle, but as its name indicates it’s only a cycle storing no heat – and I want to know where the heat goes.
Do a heat balance on the refrigerant. Look where the heat comes from, where it goes. Scrutinizing how the refrigerant works makes you miss the whole purpose of bringing work to the system. Of course, if you do not pay for it, heat won’t go from cold to warm – but the whole purpose of the fridge is to bring on work in order to reverse the balance
Same goes for the atmosphere. Earth is not an isolated system by far, lots of external energy flows in. Air will play a similar role to the refrigerant, but you don’t care – you calculate the overall balance.
You have to work your physics skiils, you have the nose too close to details and you miss therefore the larger picture. You are not alone : when I teached physics courses, students too often blindly went into calculation mode instead of making a pause and thinking about the problem.
I am more interested in you climatards explaining how Sydney’s temperature hit 45.8c… No global warming or CLIMATE CHANGE back then was there… or maybe the climate changes all the time you idiots, who are basing your stupid claims on only a few years of data with faulty computer models… but hey, if you just keep tweaking the computer models you can keep on coming up with brand new predictions.. which is how the whole scam of an industry has sucked hundreds of billions of dollars out of the global economy.